meodingu
Member

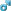 Number of posts : 270 Number of posts : 270
Pointz :
 |  Subject: Allotropes of iron Subject: Allotropes of iron  Sun Oct 24, 2010 8:27 pm Sun Oct 24, 2010 8:27 pm | |
| Allotropes of iron Iron represents perhaps the best-known example of allotropy in a metal. There are three allotropic forms of iron, known as α, γ and δ. Phase diagram of pure iron As molten iron cools down it crystallizes at 1538 °C into its δ allotrope, which has a body-centered cubic (bcc) crystal structure. As it cools further its crystal structure changes to face-centered cubic (fcc) at 1394 °C, when it is known as γ-iron, or austenite. At 912 °C the crystal structure again becomes bcc as α-iron, or ferrite, is formed, and at 770 °C (the Curie point, Tc) iron becomes magnetic. As the iron passes through the Curie temperature there is no change in crystalline structure, but there is a change in "domain structure", where each domain contains iron atoms with a particular electronic spin. In unmagnetized iron, all the electronic spins of the atoms within one domain are in the same direction; the neighboring domains point in various directions and thus cancel out. In magnetized iron, the electronic spins of all the domains are aligned, so that the magnetic effects of neighboring domains reinforce each other. Although each domain contains billions of atoms, they are very small, about 10 micrometres across.[6] Iron is of greatest importance when mixed with certain other metals and with carbon to form steels. There are many types of steels, all with different properties, and an understanding of the properties of the allotropes of iron is key to the manufacture of good quality steels. Alpha iron, also known as ferrite, is the most stable form of iron at normal temperatures. It is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[7] Above 912 °C and up to 1400 °C α-iron undergoes a phase transition from bcc to the fcc configuration of γ-iron, also called austenite. This is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.[6] Myrtle Beach Short SalesAtlanta Bankruptcy Attorney | |
|
